
The magic of everyday chemistry reflects the chemical reactions and principles that operate in our daily lives. These processes influence activities such as cooking, breathing (respiration), combustion, fermentation, photosynthesis and other physical processes, like precipitation and condensation.
Our day starts and ends with chemical reactions. Chemistry explains the world around us, from cleaning with soaps, hand wash, and detergents to cooking or baking with baking powder, producing fluffiness through carbon dioxide release.
Have you ever wondered why the role of chemistry is so important in everyday life?
Everything we see, hear, smell, taste, and touch, including ourselves, involves an intricate series of chemical reactions and interactions within our bodies. Chemical reactions cause many of the changes we observe in the world around us.
Chemistry extends beyond the confines of beakers and laboratories. Chemistry is present in every aspect of life around us.
| What is everyday chemistry? 'Everyday chemistry' refers to chemical reactions and processes that occur in daily activities such as cooking, cleaning, breathing, and energy use. |
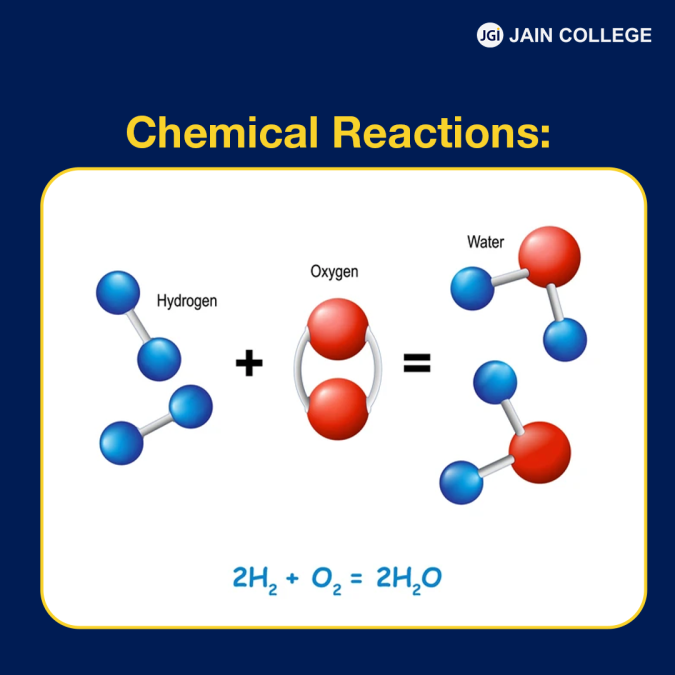
Chemical reactions can be simply defined as transformations of reactants into products.
For example: Here is a simple chemical reaction of water:
| 2H₂ + O₂ → 2H₂O[When two molecules of hydrogen (H) reacts with one molecule of oxygen (O), they give rise to two molecules of water (2H₂O).] |
Chemical reactions can be classified into several basic categories based on how substances interact and transform. Understanding these types helps explain countless processes in everyday life—from cooking food to rusting metals.
Major types of chemical reactions:
| Type of Reaction | Description | Everyday Example |
| Combination (Synthesis) | Two or more substances combine to form a single product | Formation of water (H₂ + O₂ → H₂O) |
| Decomposition | A compound breaks down into simpler substances | Baking soda releases CO₂ when heated. |
| Displacement (Single Replacement) | One element replaces another in a compound | Rusting of iron |
| Double Displacement | The exchange of ions between two compounds | Soap reacts with hard water. |
| Combustion | Substance reacts with oxygen to release energy | Burning fuels like petrol or LPG |
These reactions form the foundation of chemistry in daily life and help explain physical changes we observe around us.
Chemistry is a branch of science that deals with matter: its properties, composition, and structure, including atoms, molecules, elements, and mixtures. All matter around us is composed of chemical substances that play a vital role in our daily lives.
Our day usually starts with brushing our teeth. It is an example of chemistry. The reaction between the ingredients in toothpaste and water cleans our teeth, protects them from bacteria, and makes us feel fresh. Similarly, chemistry is present in every aspect of our lives.
Here are some examples of everyday chemistry:

Every day while washing our clothes or utensils, we use soaps and detergents. These cleaning products are made of chemical ingredients. Soaps and detergents are sodium or potassium fatty acid salts, produced from the hydrolysis of fats in a chemical reaction called saponification.

Different types of chemical substances make up most of the items we use in our kitchen on a daily basis. This list includes
In addition to cooking, we also use the following in the kitchen:
Cookware:
Does cooking also relate to chemistry?
Cooking involves a series of chemical reactions that permanently change food:
Coffee is one of the most popular beverages prepared from roasted, ground coffee beans. It is one of the most widely consumed beverages worldwide. The main chemical compounds in coffee beans include caffeine, acids, sugars, and some aromatic compounds, all of which contribute to the complex flavour of coffee. The main magic of chemistry happens during the roasting process. This is called the heat-induced browning process, primarily with the Maillard reaction (between sugars and amino acids) and caramelisation (sugar breakdown).
This is because there is a chemistry behind it.
Onion cells contain sulphur-containing compounds. As a result, when we cut onions for cooking or dressing salads, the onion cells are broken, and chemical compounds undergo chemical reactions. Accordingly, Sulfenic Acid produces a volatile gas known as Propanethiol S-oxide, which stimulates tear production by triggering the tear glands to flush out the irritant.
The human body functions like a complex chemical factory, where thousands of reactions occur within our cells, tissues, and organs every second to sustain life. Digestion, respiration, anabolism, catabolism, and enzyme reactions are some examples of major chemical reactions in the human body. Together, these chemical reactions are called metabolism. They help us acquire energy, grow, repair tissues, and maintain normal body functions.
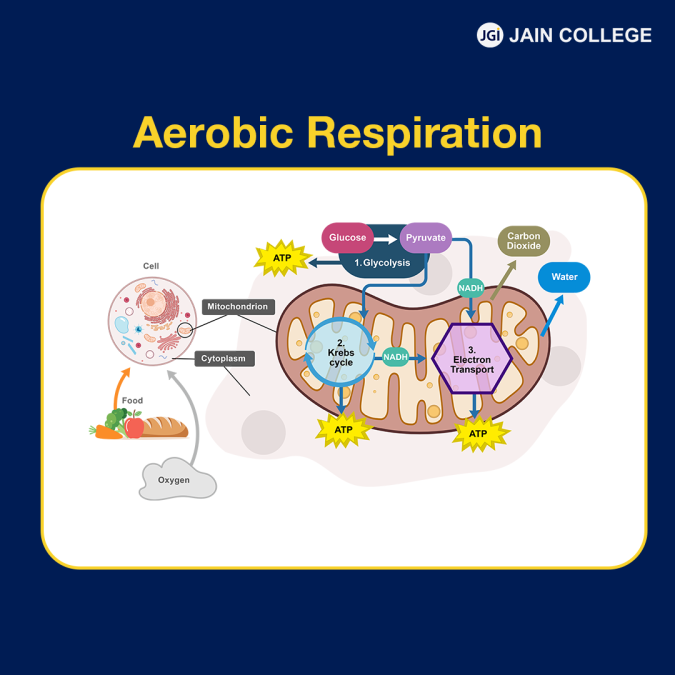
Aerobic respiration is a cellular process in which oxygen is used to release energy from glucose. It is a defining characteristic of all aerobic organisms. Aerobic respiration and photosynthesis are complementary processes in nature. In this biochemical process, glucose molecules combine with oxygen to release the energy needed for our cells to perform their functions.
Here is the chemical reaction for aerobic respiration:
| C6H12O6 + 6O2 → 6CO2 + 6H2O + energy (ATP) |
Our emotions are also governed by chemistry:
Hormones and neurotransmitters interact through chemical signals, shaping how we feel and react daily.
Fuel is the combustible substance (solid, liquid, or gas) used to generate energy for transportation, heating, and power generation.
The crude oil found under the Earth's crust is converted into gasoline, diesel, LPG, CNG, kerosene, oils, and other fuels through complex refining processes.
Fuels contain carbon (C) and hydrogen (H) as main constituents. These elements undergo chemical reactions like combustion with oxygen, and this energy is used to produce power.
Nature itself is driven by powerful chemical reactions that maintain balance on Earth.

Rusting and corrosion are both chemical processes. Rusting is a specific type of corrosion that involves the oxidation of iron. During the rusting process, an iron metal and its alloys develop a reddish or orange-brown coating, known as rust.
Rusting is a slow oxidation reaction.
Here is the chemical reaction for the rusting of iron:
| 4Fe + 3O₂ + 6H₂O → 4Fe(OH)3 (Rust) |
Photosynthesis is called "nature's chemistry lab" because it is a highly complex and efficient series of biochemical reactions.
Photosynthesis is fundamentally a series of complex chemical reactions in which all green plants with chlorophyll pigments synthesise their own nutrients with the help of solar energy, water, and carbon dioxide.
Here is the chemical equation for photosynthesis:
| 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂ |
According to the above reaction, when six molecules of carbon dioxide combine with six molecules of water, the reactants produce glucose and oxygen. In this chemical reaction, Glucose (C₆H₁₂O₆) is the main product, and oxygen (O₂) is a byproduct.
Chemistry plays a crucial role in innovation and sustainability.
Key Modern Applications
Modern chemistry is not just about discovery—it is about creating a safer, more sustainable future.
Everything is made of chemicals.
Chemistry and its chemical reactions cause many of the changes we observe in our daily lives.
Chemistry is important because it helps us understand the composition, structure, and changes of matter.
Chemicals are used in many ways in our daily lives, including in food, cleaning, washing, product creation, and construction. Chemistry plays a crucial role in various aspects of our daily lives. It is especially important in medicine, food production, and detergent manufacturing.

Everyday chemistry matters because:
Chemistry is considered a fundamental part of our everyday lives. Science encompasses chemistry beyond research, medication preparation, and cooking and boiling.
Here are some more examples to prove the importance of chemistry in everyday life.
These are a few materials that we use in our daily lives. Chemical reactions bring all these products or resources to life.
Now we can see the magic of everyday chemistry. Observe it carefully and notice the magic around us.
Chemistry is the magic that happens every day in our lives. The chemical reactions eventually happen, keeping the world perfectly balanced.
Without Chemistry, life is not possible. Chemistry connects the world through reactions and innovations that improve our lives.
From ocean waves to plant growth, chemical reactions drive it all. Master everyday chemistry to understand our world's building blocks.
We can all observe that chemistry is, overall, present in our everyday lives. Therefore, chemistry is a powerful way to better understand life.
Chemistry plays a role in every aspect of life and is not confined to laboratory settings. Everything around us is made of chemicals, and countless chemical reactions take place in our daily lives, from breathing and digestion to cooking food and cleaning our surroundings.
For more information about the magic of chemistry in our daily experiences, its phenomena, and other related articles, visit our blogs.
Yes. Respiration in humans is an example of a chemical reaction.
Glucose + Oxygen ? Carbon dioxide + Water + Energy.
Yes. Germination of seeds is a chemical process, as it involves the actions of hormones and enzymes.
This is due to the baking powder being mixed with liquid (water) and heat. It releases carbon dioxide for leavening and creates a soft, spongy texture in roti doughs.
Yes. Chemical reactions are constantly occurring in daily life. Some examples include photosynthesis, combustion, the rusting of iron, and both aerobic and anaerobic respiration.
In agriculture, chemistry and chemical reactions play a fundamental role in improving crops, developing fertilisers, and supporting various agricultural processes.

JAIN PU College, a part of the renowned JGI Group, is committed to empowering students with quality education.
Beyond academics, the college ensures its online content reflects the same standard of excellence. Every blog and article is meticulously vetted and proofread by subject matter experts to ensure accuracy, relevance, and clarity. From insightful educational topics to engaging discussions, JAIN PU College's content is crafted to inform, inspire, and add value to its readers, reflecting the institution's commitment to intellectual growth and innovation.
View all Blogs